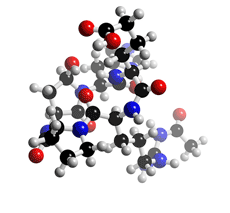
The molecular structure of Argadin
Click on the image above to interact with the 3D model of the Argadin structure
Argadin
Discovery, producing organism and structure (1,2)
Argadin was isolated from the culture broth of the fungal strain FO-7314 and identified as a chitinase inhibitor. It is a cyclic pentapeptide composed of Nψ-acetyl-L-arginine, Dproline, L-aspartic β-semialdehyde, L-histidine, and L-2-aminoadipic acid in which the aldehyde carbon of aspartic β-semialdehyde bonded to the histidyl α-amino residue. The stereochemistry of Cα and Cγ of aspartic β-semialdehyde and Cα of histidine were elucidated from the crystal structure of the argadin-chitinase complex. Argadin is the first peptide inhibitor of glycosidase showing nanomolar inhibition, and the argadin-chitinase complex revealed how the argadin backbone and side chains mimic the interactions of the enzyme with chitooligosaccharides.
Physical data
White powder. C29H42N10O9; mol wt 674.72. Sol. in acidic H2O, acidic DMSO. Slightly sol. in H2O, DMSO. Insol. in MeOH, acetone, CHCl3.
Biological activity
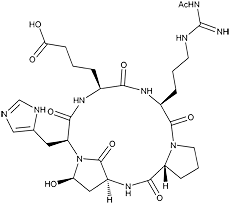
Argadin
1) Chitinase inhibition (1,2)
2) Argadin-chitinase complex and argifin-chitinase complex (2)
The structures of argifin and argadin in complex with Serratia marcescens chitinase B were
resolved (2.0 Å resolution). These structures give an unprecedented view of how peptide
based inhibitors inactivate carbohydrate-processing enzyme. For example, the carbonyl
oxygen of the histidine in argadin occupies almost the same position as the scissile oxygen
in the chitinase B-chitooligosaccharide complex and hydrogen-bonds to the catalytic acid
(Glu144), while Glu-144 makes hydrogen bonds to the guanidinium group of the arginine
side chain in argifin.
Argadin was injected into American cockroach (Periplaneta americana) larvae and compared with controls. No mortality was observed for control cases after day 1, while 60% and 38% mortality was observed for cockroaches injected with 20 µg and 2 µg of argadin, respectively.
4) Other biological activity (1)
Argadin did not inhibit the growth of tested bacteria, yeast, or fungi at 10 µg/disc (paper
disc method). Argadin also did not inhibit the growth of P388, KB, or HL-60 cells at 25
µg/ml.
References
1. [759] N. Arai et al., Chem. Pharm. Bull., 48, 1442–1446 (2000)
2. [805] D. R. Houston et al., Proc. Natl. Acad. Sci. USA, 99, 9127–9132 (2002)
3. A. Turberg et al., In “Chitin and Chitosan–Chitin and Chitosan in Life
Science” (Eds. T. Uragami et al.) pp. 440-442, Kodansha Scientific (2001)


