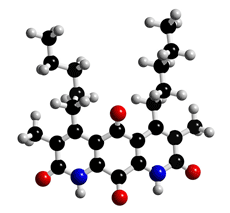
The molecular structure of Diazaquinomycin
Click on the image above to interact with the 3D model of the Diazaquinomycin structure
Diazaquinomycin
Discovery, producing organism and structure (1,2)
Diazaquinomycins were found while screening for antifolate substances in microorganisms. Diazaquinomycin A inhibited the growth of Gram-positive bacteria. 11,18 -Diacetoxydiazaquinomycin A exhibited antitumor activity against Meth-A fibrosarcoma. The total synthesis of diazaquinomycin A has been reported by two groups. The first total synthesis was achieved by Kelly et al (3).
Physical data (Diazaquinomycin A)
Red crystals. C20H22N2O4; mol wt 354.41. Slightly sol. in DMSO, MeOH, acetone, CHCl3. Insol. in H2O, hexane.
Screening method (4)
Most general microorganisms cannot incorporate folate-related compounds, but some special microorganisms such as Streptococcus sp. and Lactobacillus sp. require folate-related compounds and thus can incorporate them. Antifolates are used clinically as anticancer and antibacterial drugs. To screen the antifolate compounds, we selected a culture broth of soil isolates showing inhibitory activity against a Streptococcus sp. grown in a medium containing a limited amount of pteroate, enough amino acids, bases, and nucleosides, (except thymine and thymidine (TdR)), but lacking inhibitory activity against organisms grown in the same medium supplemented with a sufficient amount of TdR.
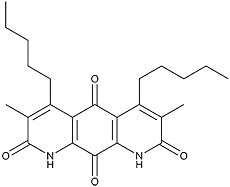
Diazaquinomycin D
Biological activity (1,5,6,)
1) Antimicrobial activities
Diazaquinomycin A inhibited the growth of Gram-positive bacteria (MIC: 3.13–50 µg/ml)
with the exception of Bacillus.
2) Cytotoxicity
IC50 = 0.86 µg/ml (Vero cells), 0.23 µg/ml (Raji cells)
3) Acute toxicity (mice i.p.)
LD50 = 100 mg/kg
4) Antitumor activity
11,18-Diacetoxydiazaquinomycin A exhibited antitumor activity
against Meth-A fibrosarcoma (10 mg/kg/day, day 1–4, T/C 141%;
100 mg/kg/day, days 1–4, T/C 175%).
Mode of action (5)
The inhibitory site of diazaquinomycin A was confirmed to be thymidylate synthase. It competitively inhibited bacterial and mammalian thymidylate synthases.
References
1. [250] S. Ōmura et al., J. Antibiot., 35, 1425–1429 (1982)
2. [267] S. Ōmura et al., Tetrahedron Lett., 24, 3643–3646 (1983)
3. T. R. Kelly et al., Tetrahedron Lett., 29, 3545–3546 (1988)
4. [326] S. Ōmura et al., J. Antibiot., 38, 1016–1024 (1985)
5. [327] M. Murata et al., J. Antibiot., 38, 1025–1033 (1985)
6. [412] K. Tsuzuki et al., J. Antibiot., 42, 727–737 (1989)


