The molecular structure of Hymeglusin F-244
Click on the image above to interact with the 3D model of the Hymeglusin F-244 structure
Hymeglusin
Discovery, producing organism and structure (1-4)
Specific inhibitors of mevalonate biosynthesis were screened with an intact mammalian cell assay (Vero cells). Hymeglusin (1233A, F-244 or L-659,699) was isolated from a culture broth of the fungal strain F-244 and found to be an inhibitor of Vero cell growth in MEM medium containing 2% calf serum, but not in the above medium supplemented with 1 mM mevalonate (2).
The absolute configuration was determined by Chiang et al (3). The total synthesis of hymeglusin has been reported by several groups. The first total synthesis was reported by Mori et al (5).
Physical data (1)
White amorphous powder. C18H28O5; mol wt 324.19. Sol. in MeOH, EtOH, CH3CN, EtOAc, CHCl3. Insol. in H2O, hexane. Store at –20oC.
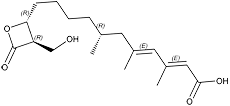
Hymeglusin F-244
Biological activity (1, 6-9)
1) Specific and irreversible inhibition of HMG-CoA synthase in vitro (6–8) and in vivo (9)
2) Antimicrobial activity (1)
Hymeglusin showed antifungal activity against Candida albicans (MIC: 12.5 µg/ml),
Penicillium herquei (25) and Pyricularia oryzae (6.25).
Importance of β-lactone geometry for specific HMG-CoA synthase inhibition (10–15)
Two kinds of natural β-lactones have been reported; (2R,3R)-β-lactone as a HMG-CoA synthase
inhibitor and (2S,3S)-β-lactone as a lipase (esterase) inhibitor. During our synthetic
study of β-lactone analogs, four optically active DU-6622s were synthesized to discriminate
between the inhibitory activity of the two. Consequently, (2R,3R)-β-lactone was found to
be responsible for specific inhibition of HMG-CoA synthase, whereas (2S,3S)-β-lactone
was responsible for pancreatic lipase inhibition.
Biosynthesis (16)
References
1. [382] H. Tomoda et al., J Antibiot., 41, 247–249 (1988)
2. D. C. Aldridge et al., J. Chem. Soc., (C) 3888–3891 (1971)
3. Y. C. Chiang et al., J. Org. Chem., 53, 4599–4603 (1988)
4. [440] H. Kumagai et al., J. Antibiot., 43, 397–402 (1990)
5. K. Mori et al., Liebigs Ann. Chem., 1991, 1057–1065 (1991)
6. [366] S. Ōmura et al., J. Antibiot., 40, 1356–1357 (1987)
7. [377] H. Tomoda et al., Biochim. Biophys. Acta., 922, 351–356 (1988)
8. [503] H. Tomoda et al., J. Antibiot., 46, 872–874 (1993)
9. [523] H. Nagashima et al., Life Sciences, 52, 1595–1600 (1993)
10. [661] H. Tomoda et al., J. Org. Chem., 62, 2161–2165 (1997)
11. [483] T. Sunazuka et al., J. Antibiot., 45, 1139–1147 (1992)
12. [538] H. Hashizume et al., Chem. Pharm. Bull., 42, 512–520 (1994)
13. [543] H. Hashizume et al., Chem. Pharm. Bull., 42, 1272–1278 (1994)
14. [544] H. Hashizume et al., Heterocycles, 38, 1551–1571 (1994)
15. [551] H. Hashizume et al., Chem. Pharm. Bull., 42, 2097–2107 (1994)
16. [480] H. Kumagai et al., J. Antibiot., 45, 563–567 (1992)


