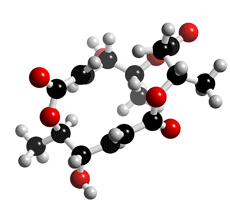
The molecular structure of Macrosphelide E
Click on the image above to interact with the 3D model of the Macrosphelide E structure
Macrosphelide
Discovery, producing organism and structure (1-8)
Macrosphelides A-D, J and K were isolated from a culture broth of the fungal strain FO- 5050 and recognized as cell-adhesion inhibitors (1-5). It was reported by Numata et al that macrosphelides E-I, L were isolated from another fungal strain (6-8). The total syntheses of macrosphelides have been reported by several groups (4).
Physical data (Macrosphelide A) (1-5)
Colorless needles. C16H22O8 ; mol wt 342.13. Sol. in CHCl3, MeOH, EtOAc. Insol. in hexane.
Biological activity
1) Inhibition of cell adhesion (1,3)
Macrosphelide (MS) A showed potent inhibitory activity against cell adhesion of human
leukemia cell (HL-60) to LPS-activated human umbilical vein endothelial cell (HUVEC),
with an IC50 value of 3.5 µM. The IC50 values of macrosphelides B, C and D were 36, 67.5
and 25 µM, respectively. The IC50 values of both macrosphelides J and K were greater than
100 µg/ml. Macrosphelide A and B did not show any cytocidal activities at a concentration
of 100 µg/ml.
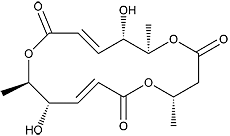
Macrosphelide E
2) MSB suppressed metastasis through inhibition of adhesion of sLex/E-selectin
molecules (9).
MSB inhibits adhesion of sialyl Lewisx (sLex)-expressing HL-60 cells to LSP-activated
(E-selectin-expressing) HUVECs in vitro. When MSB was administered to B16/BL6 mice
at 20 mg/kg/day, lung metastatic nodules of B16/BL6 mouse melanoma cells (B16/BL6
cells) were significantly decreased (T/C = 45%). Flow cytometry analysis showed that
B16/BL6 cells expressed high levels of sLex antigens. Under in vitro conditions, B16/BL6
cells demonstrated a greater degree of adhesion to LSP-activated HUVECs, but adhesion
was significantly inhibited by MSB and sLex antibodies. Combined therapy of MSB and
cisplatin (CDDP) induced remarkable lung metastasis inhibition without the adverse effects, of CDDP to the host.
References
1. [597] M. Hayashi et al., J. Antibiot., 48, 1435–1439 (1995)
2. [602] S. Takamatsu et al., J. Antibiot., 49, 95–98 (1996)
3. [683] S. Takamatsu et al., J. Antibiot., 50, 878–880 (1997)
4. [680] T. Sunazuka et al., J. Am. Chem. Soc., 119, 10247–10248 (1997)
5. [831] A. Fukami et al., J. Antibiot., 52, 501-504 (1999)
6. A. Numata et al., Tetrahedron Lett., 38, 8215–8218 (1997)
7. A. Numata et al., J.Chem. Soc., Perkin Trans. I., 3046–3053 (2001)
8. A. Numata et al., J. Antibiot., 55, 147–154 (2002)
9. [801] A. Fukami et al., Bioche. Biophys. Res. Comm., 291, 1065–1070 (2002)


