The molecular structure of Madindoline A
Click on the image above to interact with the 3D model of the Madindoline A structure
Madindoline
Discovery, producing organism and structure (1,2)
Madindolines were isolated from a culture broth of the actinomycete strain K93-0711 and found to be selective growth inhibitors of IL-6-dependent cells (MH-60) (1). Madindoline B (3aR, 8aS, 2’S) is a stereoisomer of madindoline A (3aR, 8aS, 2’R) (2,3). The total syntheses of madindolines have been reported by two groups 1) First total synthesis (3) and 2) Second total synthesis (4).
Physical data (Madindoline A) (2)
Light yellow needles. C22H27NO4 ; mol wt 370.20. Sol. in MeOH, EtOH, CHCl3. Insol. in hexane.
Biological activity (1)
1) Growth inhibition of the IL-6-dependent MH60
cells
Madindolines A and B (MDL-A, B) showed
potent growth inhibitory activity against IL-6
dependent MH60 cells with IC50 values of 8 and 30 µM, respectively. Madindolines did not show antimicrobial
or cytocidal activity at 1000 µg/ml and 100µg/ml, respectively.
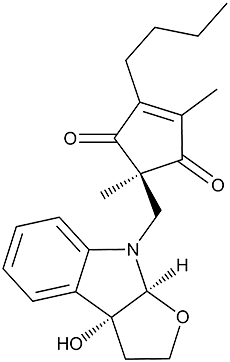
Madindoline A
2) Selectivity of madindoline A
Selectivity against some cytokines was examined in
cytokine-dependent or -sensitive cell lines. MDL-A inhibited growth of IL-6 dependent
MH60 cells at 68 µM, but did not influence other cytokine activity.
3) Inhibitory mechanism of madindoline A (5)
IL-6 has three topological binding sites (sites I, II, and III), and gp130 has two binding
sites (sites 1 and 2); IL-6 first binds to the IL-6 receptor (IL-6R) at site I, IL-6 then
binds to site I of the first gp130 at site II, forming a trimeric IL-6/IL-6R/gp130 complex.
The trimeric complex then induces homodimerization of gp130 and forms a hexameric
complex, activating the JAK/STAT signal transduction cascade.
MDL-A binds to gp130 and inhibits actions of IL-6 without inhibiting formation of the
trimeric complex. Therefore, the MDL-A mechanism of action involves binding to gp130
site 2, the site for IL-6 site III, and inhibiting gp130 homodimerization, resulting in inhibition
of IL-6 activity.
References
1. [647] M. Hayashi et al., J. Antibiot., 49, 1091–1095 (1996)
2. [688] S. Takamatsu et al., J. Antibiot., 50, 1069–1072 (1997)
3. [745] T. Sunazuka et al., J. Am. Chem. Soc., 122, 2122-2123 (2000)
4. [799] T. Hirose et al., Organic Letters 4, 501-503 (2002)
5. [813] M. Hayashi et al., Proc. Natl. Acad. Sci. USA, 99, 14728-14733 (2002)


