The molecular structure of Nanaomycin A
Click on the image above to interact with the 3D model of the Nanaomycin A structure
Nanaomycin
Discovery, producing organism and structure (1-6)
While screening for antibiotics more active against Mycoplasma gallisepticum than against bacteria, nanaomycins A, B, C, D and E were isolated from a culture broth of the actinomycete strain OS-3966. Nanaomycin A was found to possess potent inhibitory activity against fungi. The total syntheses of nanaomycins have been reported by many groups. The first total synthesis of nanaomycins A and D was reported by Li et al (7).
Physical data (Nanaomycin A)
Orange needles. C16H14O6; mol wt 302.08. Sol. in MeOH, EtOH, CHCl3, EtOAc.
Biosynthesis (3,8–11)
Nanaomycins are biosynthesized from eight acetate units via “polyketide” and are converted to D→A→E→B by enzymatic and non-enzymatic systems.
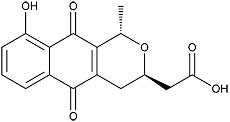
Nanaomycin A
Biological activity (2,4,13,14)
1) Antimicrobial activity of nanaomycin A (2,4).
2) Therapeutic effect of nanaomycin A against Trichophyton mentagrophytes infection in guinea pigs (12)
3) Mode of action (13,14)The quinone antibiotics, nanaomycins, are reduced by the respiratory chain-linked NADH or flavin dehydrogenase of the organism. The reduced forms of nanaomycins are quickly autooxidized by molecular oxygen producing O2—. The ability to produce O2— is related to the antimicrobial activity of nanaomycins.
---
Nanaomycin is commercially available as an antifungal agent for animals.
References
1. [74] S. Ōmura et al., J. Antibiot., 27, 363–365 (1974)
2. [96] H. Tanaka et al., J. Antibiot., 28, 860–867 (1975)
3. [97] H. Tanaka et al., J. Antibiot., 28, 868–875 (1975)
4. [100] H. Tanaka et al., J. Antibiot., 28, 925–930 (1975)
5. [109] S. Ōmura et al., J. Chem. Soc., Chem. Commun., 320–321 (1976)
6. [157] M. Kasai et al., J. Antibiot., 32, 442–445 (1979)
7. T. T. Li et al., J. Am. Chem. Soc., 100, 6263–6265 (1978)
8. [184] C. Kitao et al., J. Antibiot., 33, 711–716 (1980)
9. [211] S. Ōmura et al., J. Biochem., 90, 291–293 (1981)
10. [212] S. Ōmura et al., J. Biochem., 90, 355–362 (1981)
11. [251] H. Tanaka et al., J. Antibiot., 35, 1565–1570 (1982)
12. [197] K. Kitaura et al., Jpn. J. Antibiot., 33, 728–732 (1980)
13. [196] H. Marumo et al., J. Antibiot., 33, 885–890 (1980)
14. [247] M. Hayashi et al., J. Antibiot., 35, 1078–1085 (1982)


