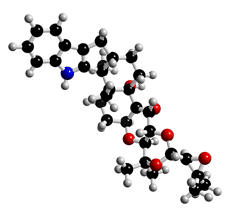
The molecular structure of Terpendole A
Click on the image above to interact with the 3D model of the Terpendole A structure
Terpendole
Discovery, producing organism and structure (1-6)
Terpendoles were isolated from a culture broth of the fungal strain FO-2546 and found to be inhibitors of acyl-CoA:cholesterol acyltransferase (ACAT). The taxonomic study of the producing organism led us to establish a new genus of Albophoma yamanashiensis (1,2). Terpendoles have a common indoloditerpene moiety. Structurally-related known compounds, paspaline (3) and emindole SB (4), were also produced and isolated from the same strain. The relative stereostructures were determined by NOE experiments and X-ray crystallographic analysis of terpendoles D and E (5,6).
Physical data (Terpendole A) (3)
White powder. C32H41NO6; mol wt 535.29. Sol. in MeOH, EtOAc, CHCl3, DMSO. Insol. in H2O, hexane.
Biological activity (2,6-8)
Terpendole A
1) Enzyme assay for ACAT inhibition
ACAT inhibitory activity was tested in an enzyme assay using
rat liver microsomes. An additional prenyl residue at the diterpene moiety is responsible
for potent ACAT inhibition.
2) Cell assay (2)
ACAT inhibitory activity was evaluated in a cell assay using J774 macrophages. Cytotoxicity
(CD50) was also determined to evaluate the specificity. Among the terpendoles tested,
terpendole D was the most potent ACAT inhibitor (IC50) and had the highest specificity.
Some indoloditerpenes were reported to be tremorgens. Terpendole C was found to have tremorgenic activity in mice. It was faster acting and produced more intense tremors than the same dose of paxilline (8). It is still unclear as to whether or not other terpendoles show tremorgenic activity.
4. Inhibition of motor activation of mitotic kinesin Eg 5 by terpendole E (9)
Terpendole E was recognized as a specific M phase inhibitor. The compound inhibited both
motor and microtubule-stimulated human Eg 5 ATPase activity.
References
1. [568] T. Kobayashi et al., Mycoscience, 35, 399–401 (1994)
2. [561] X.-H. Huang et al., J. Antibiot., 48, 1–4 (1995)
3. K. Nozawa et al., In “The 29th Symposium on Chemistry of Natural
Products” pp.637–643 (1987)
4. J. P. Springer et al., Tetrahedron Lett., 21, 231-234 (1980)
5. [562] X.-H. Huang et al., J. Antibiot., 48, 5–11 (1995)
6. [586] H. Tomoda et al., J. Antibiot., 48, 793–804 (1995)
7. [659] S. C. Munday-Finch et al., J. Agricult. Food Chem., 45, 199–204 (1997)
8. R. J. Cole et al., Can. J. Microbiol., 20, 1159-1162 (1974)
9. J. Nakazawa et al., Chem. Biol., 10, 131-137 (2003)


