The molecular structure of Vineomycin A1
Click on the image above to interact with the 3D model of the Vineomycin A1 structure
Vineomycin
Discovery, producing organism and structure (1-3)
While screening for new antibiotics in actinomycetes, vineomycins A1, A2, B1 and B2 were isolated from a culture broth of the actinomycete strain OS-4742T. These compounds are active against Gram-positive bacteria and the Sarcoma 180 solid tumor in mice. Vineomycin A1 (P-1894B) also possesses potent inhibitory activity against collagen prolyl hydroxylase. The total synthesis of vineomycin B2 has been reported by several groups. The first total synthesis was reported by Danishefsky et al (4).
Physical data (Vineomycin B2) (1–3)
Yellow powder. C49H58O18; mol wt 934.36. Sol. in MeOH.
Biological activity (2,4,5)
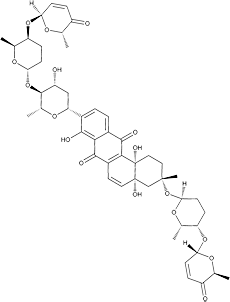
Vineomycin A1
1) Antimicrobial activity
2) Anti-tumor activity
When vineomycin A1 (50 mg/kg) was administered i.p. once a day after transplantation of sarcoma 180 cells, the tumor size (T/C) on the 7th day was 0.13.
Biosynthesis (5)
The labeling experiments with both [1-13C]- and [1,2-13C2] sodium acetate followed by 13CNMR spectroscopy revealed that the benz[a]anthraquinone chromophore of A1 originated from a decaketide metabolite by decarboxylation of the carboxyl end and that of B2 was formed via C-C bond cleavage of A1.
---
Vineomycin A1 is commercially available as a biochemical reagent.
References
1. [138] S. Ōmura et al., J. Antibiot., 30, 908–916 (1977)
2. [210] N. Imamura et al., Chem. Pharm. Bull., 29, 1788–1790 (1981)
3. [220] N. Imamura et al., J. Antibiot., 34, 1517–1518 (1981)
4. S. Danishefsky et al., J. Am. Chem. Soc., 107, 1285–1293 (1985)
5. [239] N. Imamura et al., J. Antibiot., 35, 602–608 (1982)


